Is Agno3 Nacl Agcl Nano3 an Oxidation Reduction Reaction
Click here to get an answer to your question The reaction AgNO3aq NaClaq AgCls NaNO3aq is an _____ reaction. When solutions of AgNO3 and NaCl react the balanced molecular equation is.

Type Of Reaction For Agno3 Nacl Agcl Nano3 Youtube
Ag 1 Cl -1.
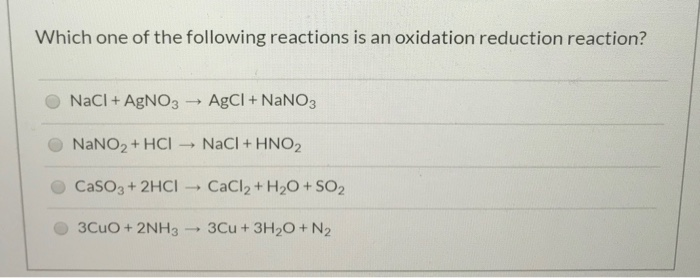
. O AgNO3 NaCl AgCl NaNO3 O Al2SO43 6KOH 2AlOH3 3K2SO4 O KOH HNO3 - H2O KNO3 O N2 O2 2NO CaCl2 Na2SO4 CaSO4 2Naci data to save Last check Question. A CaCl2 Na2SO4 ----- CaSO4 2NaCl B KOH HNO3 ---- H2O KNO3 C N2 O2 ---- 2NO D AgNO3 NaCl ---- AgCl NaNO3 E Al2 SO43 6KOH ---. A-100 years b-10000 years c-100000 years d-1000 years.
AgNO3 NaCl AgCl NaNO3 c. None of these c. Consider the equations A B C and D.
Answer to Is the reactionNaClaq AgNO3aq NaNO3aq AgClsan oxidation-reduction reaction. There is no change in the oxidation numbers hence it is not an oxidation-reduction reaction. Ag 1 N 5 O -2.
Estimate the temperature where Delta G 0 for the following reaction Given. Neither oxidation nor reduction. AgNO 3 aq NaCl aq AgCl s NaNO 3 aq Both silver nitrate and sodium chloride solutions are colourless solutions.
AgNO 3 aqNaClaqAgClsNaNO 3 aq Above reaction is. Which of the following is an oxidation-reduction reaction. In this tutorial we will learn about different aspects of this reaction such as precipitating and required concentrations for precipitation.
AgNO3 NaCl AgCl NaNO3 c. Au cours du processus doxydation le substrat gagne en oxygène et perd ses électrons. Correct option is C Double displacement reactions may be defined as the chemical reactions in which one component each of both the reacting molecules is exchanged to form the products.
302 203 C. See the answer Which of the following is an oxidation-reduction reaction. Which of the following is an oxidation reduction reaction.
AgCl NaNO 3. La réaction doxydoréduction est laddition dune oxydation et dune réduction de manière à simplifier les électronsLéquation dune oxydoréduction sécrit de la manière suivante. N2 O2 2 NO d.
KI K 1 In the reaction 3Cl2 6NaOH 5NaCl NaClO3 3H20 Cl2 undergoes A. NaCl AgNO 3 AgCl NaNO 3. 4Na O2 2Na20 B.
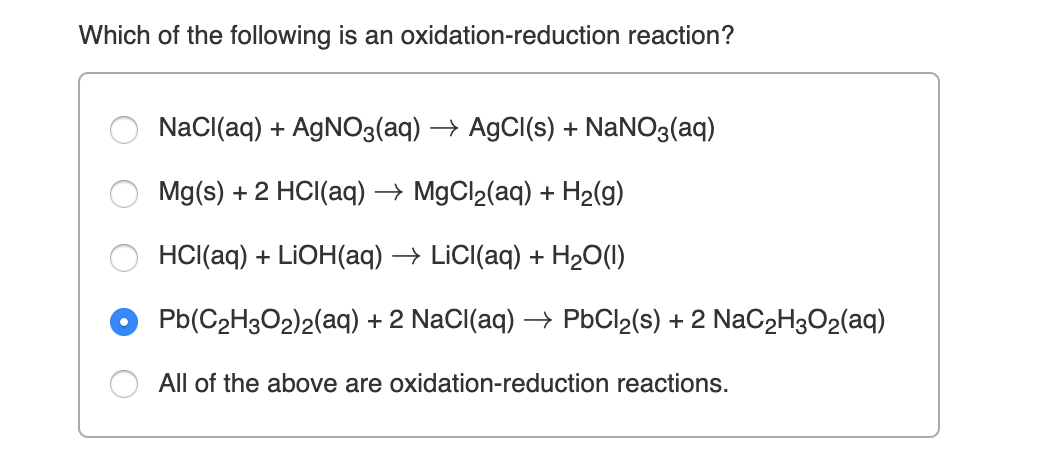
Chemistry questions and answers. HCl aq NaOH aq H2O l NaCl aq b. Which of the following is an oxidation-reduction reaction.
NaClAgNO3NaNO3AgCl is not a oxidation-reduction reaction because there is no change in oxidation state of any element. Spanish 02032021 0220. How long is radioactive waste from nuclear plants radioactive.
On the right side. Which is an oxidation-reduction reaction. Cu s 2AgNO3 aq Cu NO32 aq 2Ag s c.
Oxidation numbers of the individual element on the product side. OKOH HNO3 H2O KNO3 ON2 O2 2NO AgNO3 NaCl AgCl NaNO3 CaCl2 Na2SO4 CaSO4 2NaCl Al2SO43 6KOH 2AlOH3 3K2504 Question 3 Calculate the molar mass of magnesium chloride MgCl 2. That means AgNO 3 and NaCl is a precipitation reaction.
Which balanced equation represents a redox reaction. AgNO3 aq HCl aq AgCl s HNO3 aq d. G of silver nitrate when it is mixed with an excess of sodium chloride.
NaCl AgNO 3. Since there is an exchange of ions between the reactants it is a double displacement reaction. NaClAgNO3NaNO3AgCl is not a oxidation-reduction reaction because there is no change in oxidation state of any element.
A AgNO3 NaCl AgCl NaNO3 B Cl2 H2O HClO HCl C CuO CO CO2 Cu D NaOH HCl NaCl H2O Which two reactions are redox. 1 agno3 nacl agcl nano3. Which of the following is an oxidation-reduction reaction.
Double displacement reactions are those where two compounds react by exchanging ions to produce two new compounds. N2 O2 2 NO d. Indica si la palabra tiene diptongo con D o hiato con.
AgNO3 NaCl AgCl NaNO3 D. In double replacement reactions the positive ions exchange negative ion. Questions in other subjects.
Na 1 Cl -1. In the reaction of silver nitrate with sodium chloride how many grams of silver chloride will be produced from 100. Delta H-176 and Delta S -2845 JK NH3 HCl -- NH4Cl.
IS NaClAgNO3-AgClNaNO3 A REDOX REACTION. AgNO3aq NaClaq AgCls NaNO3aq How much AgCl is produced when 310 g. Oxidation-reducti condyyyy4933 condyyyy4933 03062020 Chemistry College answered The reaction AgNO3aq NaClaq AgCls NaNO3aq is an _____ reaction.
A AgNO3 NaCl AgCl NaNO3 B Cl2 H2O HClO HCl C CuO CO CO2 Cu D NaOH HCl NaCl H2O Which two reactions are redox. Is a redox reaction. 2 HCl aq CaCO3 s H2O l CaCl2 aq CO2 g Chemistry 1 Answer anor277 Dec 6 2017 Only b.
Précipitation nest pas un processus doxydation. Ox 1 Réd 2 Ox 2 Réd 1 avec Ox 1 Réd 1 et Ox 2 Réd 2 des couples oxydant-réducteur. Which of the following is an oxidation-reduction reaction.
In this video we determine the type of chemical reaction for the equation AgNO3 NaCl AgCl NaNO3 Silver Nitrate and Sodium Chloride. En biochimie et notamment à propos de la synthèse des molécules prébiotiques les réactions se. Oxidation numbers of the individual element on the reactant side.
NaClAgNO3NaNO3AgCl nest pas une réaction doxydo-réduction car il ny a pas de changement détat doxydation daucun élément. KOH HNO3 Question. During this reaction the cations and anions of two different compounds switch places forming two entirely different compounds.
Oxidation involves the LOSS of electrons OIL. AgNO3 NaCl AgCl NaNO3 c. AgNO3 NaCl AgCl NaNO3.
AgNO3aq NaClaq AgCls NaNO3aq A 1079 g B 1699 g C 844 g D 0589 g E 589 g. Both oxidation and reduction D. This question has multiple correct options A precipitation reaction B double displacement reaction C combination reaction D redox reaction Medium Solution Verified by Toppr Correct options are A and B This reaction is both precipitation reaction as well as double displacement reaction.
CaCl2 Na2SO4 CaSO4 NaCl b. The equation for the reaction is below. Lequel des processus suivants nest pas une réaction doxydation.
Which of the following is an oxidation-reduction reaction. KOH HNO3 This problem has been solved. OKOH HNO3 H2O.
Solved Which Of The Following Is An Oxidation Reduction Chegg Com
Type Of Reaction For Agno3 Nacl Agcl Nano3 Youtube

Solved Which One Of The Following Reactions Is An Oxidation Chegg Com
Comments
Post a Comment